Important FDA Statement about Dangerous Substance Wrongly Being Used by Patients for Opioid Addiction

FDA issues statements about the deadly risks associated with the use of kratom for opioid withdrawal symptoms.
On Nov. 14, 2017, the Food and Drug Administration (FDA) issued a public health advisory to address concerns about the deadly risks associated with a botanical substance called kratom for treating opioid withdrawal. The FDA says patients wrongly believe they can use kratom to treat opioid withdrawal symptoms, but there is no reliable evidence to support its use as a treatment for opioid use disorder.
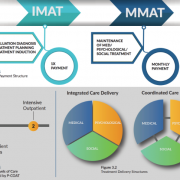
The statement goes on to reinforce the FDA’s commitment to expanding the development and use of therapies to support medication-assisted treatment (MAT treatment) and emphasizes the importance of patients utilize treatments that are proven to be safe and effective.
To access the full press release from the FDA click here.
If you have questions about the process of medication-assisted treatment, please call AOC at 330-259-4849, or e-mail us for more information or to schedule an appointment.
Addiction Outreach Clinic (AOC) provides an outpatient opioid addiction treatment program that includes Suboxone® medication-assisted treatment to prevent cravings and withdrawal symptoms combined with a once-a-month behavioral counseling session to support our patients’ recovery efforts and help them get their lives back. Since 2007, AOC has helped thousands of patients on their path to recovery.
Please read more about AOC, or call us at 330-259-4849, or email to schedule an appointment with a drug addiction specialist – it’s fast, easy and confidential.





Leave a Reply
Want to join the discussion?Feel free to contribute!